Thought Leadership - 03.02.2021
Article: How To Calculate Viral Vector Yields – A Critical Component Of The “Make vs Buy” Analysis
Calculating viral vector yields is essential to determine how much drug product is needed for a specific gene therapy. This calculation will determine either how much internal manufacturing capacity is needed or how much it may cost to manufacture at an external contract development and manufacturing company (CDMO). This analysis is critical for any gene therapy organization looking to scale from preclinical to clinical to commercial.
Years of industry experience has led to the development of a valuable yield calculation tool that can serve as a vital prerequisite of the “make vs buy” analysis, which compares the feasibility and cost of building internal manufacturing capacity versus using an external CDMO. The calculation results reveal if the process is robust enough to meet the market demand of the target patient population. If the calculation reveals that the yields are very low compared to the target population size, then the process may need to be refined before attempting to scale up. If it is ready to be scaled up, valuable insights can be gained in determining how much facility capacity, and therefore capital, will be required to proceed with commercialization.
What to Consider for the Calculation
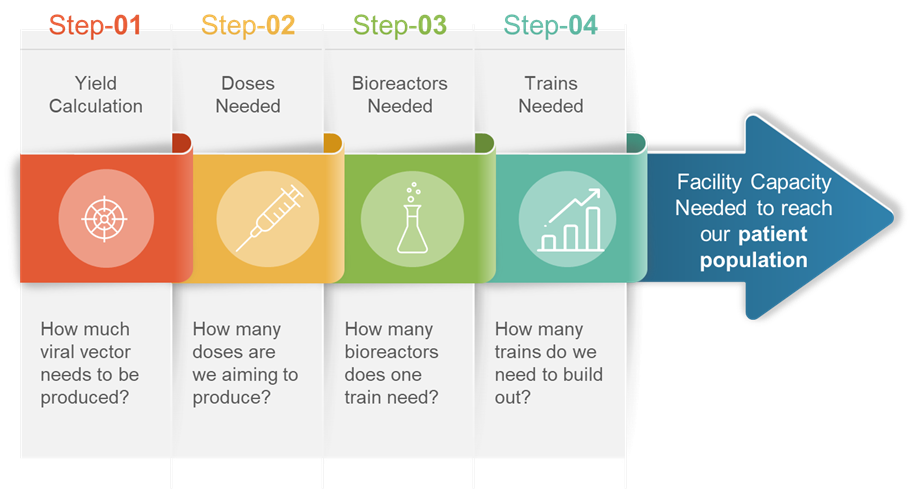
This tool evaluates critical factors to determine the dosing and manufacturing yield, which ultimately results in the number of target patients that can be served per year.
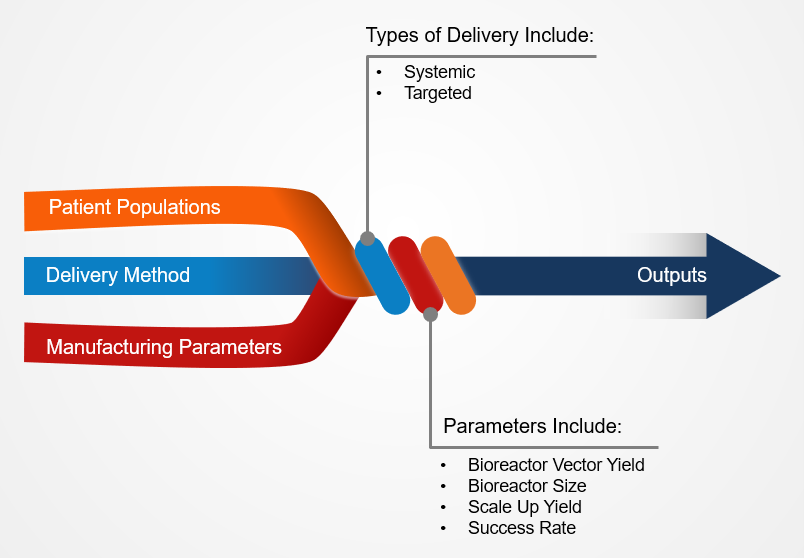
The importance of partnering with and leveraging the academic and scientific teams throughout this process cannot be understated. In order to perform this calculation, the technical operations team and the academic scientific team review current laboratory process trends and yields. The technical operations team will assess several factors of the current process capabilities, including the patient population, delivery method, how much vector is required per dose per patient, upstream and downstream ratio, and current and future bioreactor sizes. Insights into the scale up yield and the manufacturing success rate are also valuable considerations. A key unknown at this stage is often how the product will scale up—will the vector yield be linear through process scale up or will the yield taper? If the product has already been scaled up, providing the information and results from those processes is key to obtaining a more accurate result.
The target population and delivery method of the therapy is a critical piece of this calculation, each impact the dosing size. For this reason, the calculation tool is adaptable to allow the manufacturing process to account for who is receiving the dose.
Additional key factors, such as any potential loss in yield when the process is transitioned from the bench or how much will need to be taken for sampling, should also be accounted for. Industry standards should also be leveraged and assumptions on the success rate of the product and process once it is moved to the manufacturing floor should be considered.
The Calculation
The more data the team has to work with, the more accurate the calculation results will be. However, if information is not available, the team is able to utilize benchmarks that reflect the profile of the product.
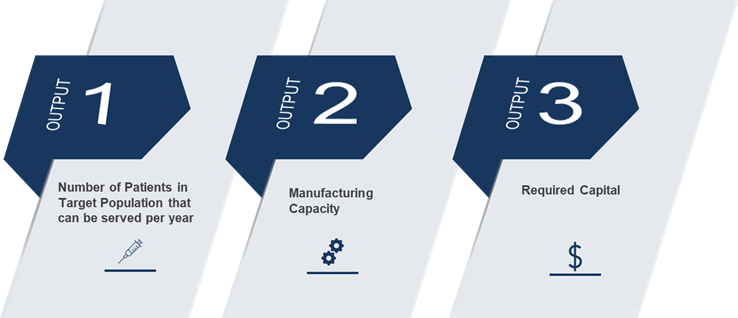
These critical outputs lay the foundation for understanding facility capacity. The actual outputs of the calculation are the total viral vector needed to be produced per year, which determines the number of doses required. Once the number of doses is calculated, the number of bioreactors and trains can be determined and, ultimately, facility capacity.
Looking Towards Scale up and Commercialization
Once your innovation has reached a pivotal point in the scale up process and you are looking to move forward with commercialization, there are several assessments to perform to better understand your manufacturing capability based on target patient population and manufacturing yields. As you prepare to manufacture at a large scale, performing the yield calculation can determine critical factors, such as the required number of doses, bioreactors, trains, manufacturing yield and facility capacity. Understanding the required facility capacity is a prerequisite step in conducting a “make vs buy” analysis of your scale up operation.